OUR PIPELINE
Redefining cancer treatment
Alterome is developing several next generation, small molecule targeted therapies for the treatment of cancer. Our pipeline features two clinical-stage programs currently in Phase 1/1b, ALTA2618, a mutation-selective inhibitor for AKT1 E17K driven cancers, and ALTA3263, an oral pan-KRAS ON-state inhibitor that targets >90% of KRAS mutations in cancer.
ALTA2618 is an orally bioavailable, mutant-selective, and covalent allosteric inhibitor of AKT1 E17K.
AKTive-001 (NCT06533059) is a Phase 1/1b multiple cohort trial of ALTA2618 in patients with advanced solid tumors with AKT1 E17K mutation.
ALTA3263 is an orally bioavailable KRAS isoform-selective inhibitor with potent ON state inhibition designed to target >90% of KRAS mutations.
The 3263-001 study [NCT06835569] is a Phase 1/1b multiple cohort trial of ALTA3263 in patients with advanced solid tumor malignancies with KRAS mutations.
Portfolio of validated oncogenic drivers with clear paths to early clinical signals
Our deep expertise in small molecule, structure-guided oncology drug discovery enables us to develop mutation and isoform-selective inhibitors against validated oncogenes. Our sense of urgency and commitment to patient benefit pave a clear path to early clinical signals.
PROGRAMS

Expertise in small molecule drug discovery
Structure & physics-based drug design

Validated oncogenic alterations
AKT1 E17K
KRAS MutationS

Maximizing therapeutic index
Mutation & isoform-selective strategies
AKT1 E17K
AKT1 E17K mutant-selective inhibitor
The PTEN/PI3K/AKT pathway controls essential cellular processes spanning growth, survival, and metabolism. Pathway alterations lead to a variety of malignancies. Among these alterations is AKT1 E17K, a clinically validated oncogene that drives ~2% of all cancers, including breast, endometrial, and prostate cancers. Precision therapy options for AKT1 E17K cancer patients have limited durability. We developed ALTA2618, an orally bioavailable, mutant-selective, and covalent allosteric inhibitor of AKT1 E17K.
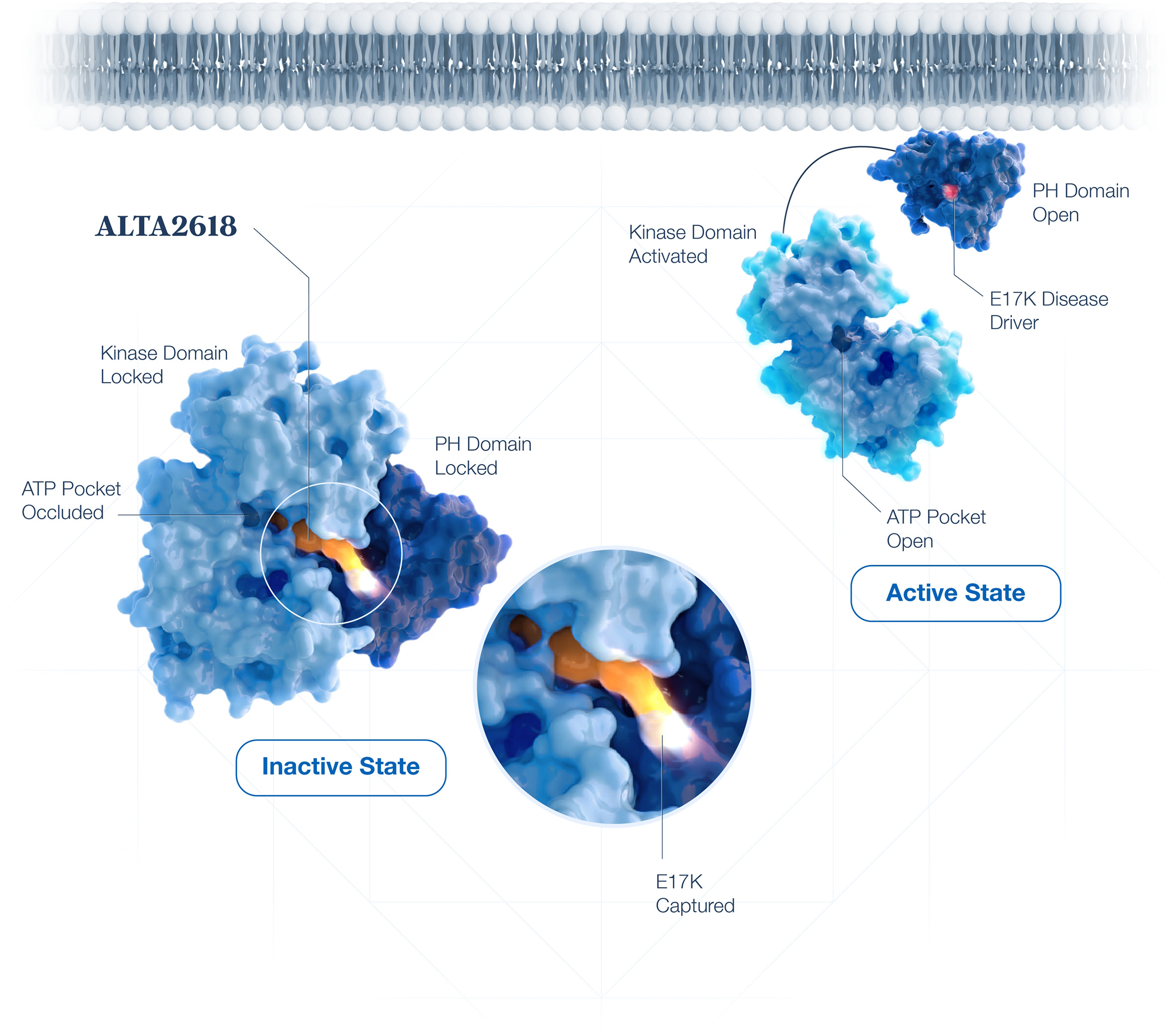
ATP-Competitive Inhibitors of AKT1
Oncogenic AKT1 E17K is kept “open” by the E17K alteration. ATP-competitive inhibitors target the activated kinase domain requiring high concentrations to compete with cellular ATP. These concentrations inhibit wild type AKT, leading to unintended toxicities.
Covalent Allosteric Inhibitors of AKT1 E17K
ALTA2618 overcomes these limitations through covalent capture of the disease-driving E17K. This strategy avoids inhibition of wild type AKT and spares patients on-target toxicities. This mutant-selective approach enables complete target coverage.
AKT1 E17K clinical trial
Study sites are currently enrolling patients with advanced solid tumors with an AKT1 E17K mutation. The purpose of this study is to characterize the safety and tolerability of ALTA2618 in adults with AKT1 E17K-mutant advanced solid tumors.
For patients and caregivers to learn more about the AKTive-001 study, please visit our trial site.
Please contact clinical.trials@alterome.com for more information.
KRAS
KRAS isoform selective
KRAS is a central regulator of multiple cellular signal transduction pathways and is mutated in >20% of all cancers. KRAS was once considered an “undruggable” target, but first-generation medicines achieved a breakthrough by targeting the OFF state of the KRAS G12C mutant. The narrow scope of mutational coverage and suboptimal properties of first-generation medicines have limited durability and poor tolerability for patients. We developed ALTA3263, a KRAS isoform selective inhibitor with improved drug-like properties and ON state inhibition to target >90% of KRAS mutations.
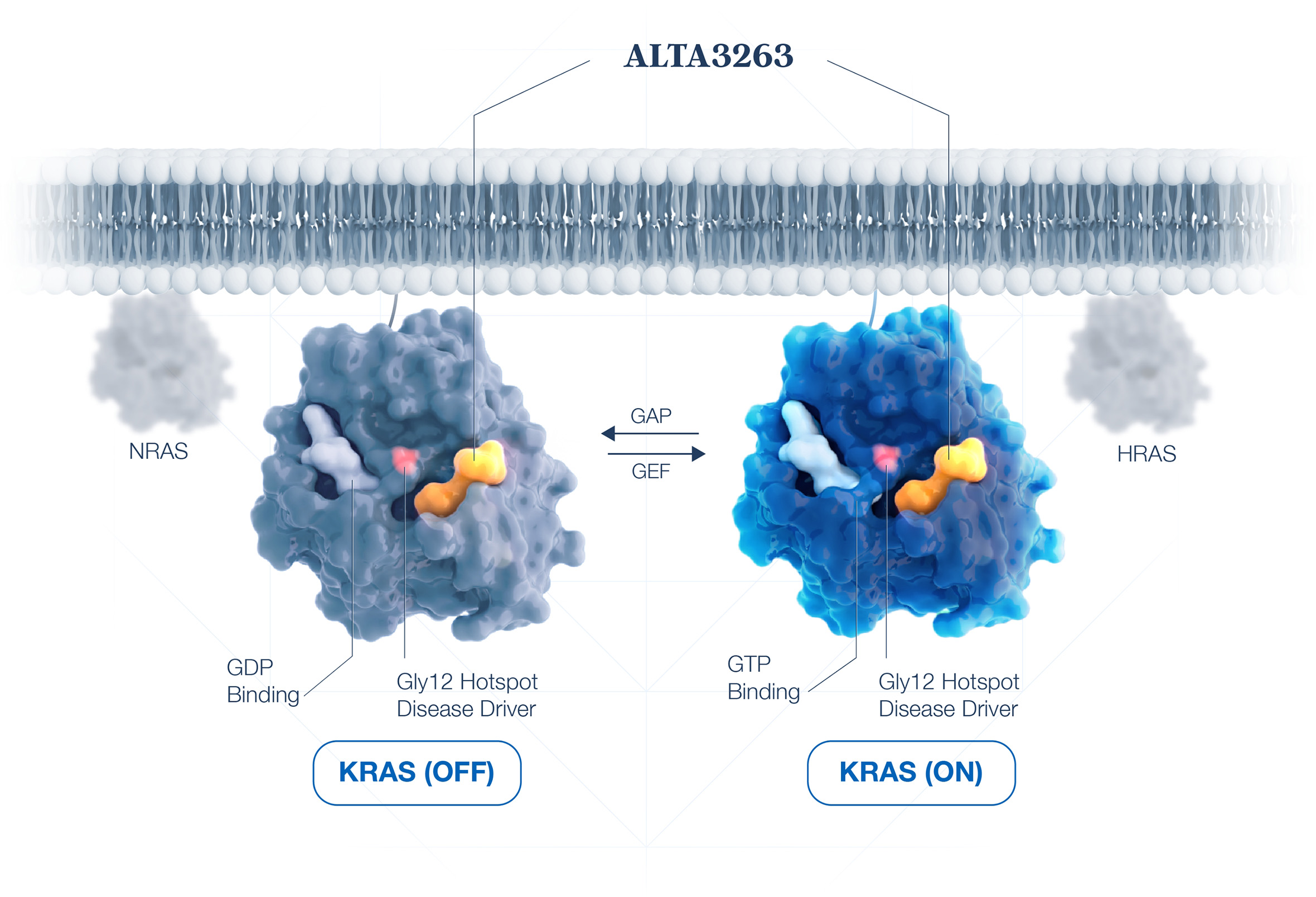
Defining KRAS inhibition
We have overcome multiple KRAS drug design hurdles to deliver a highly differentiated KRAS isoform-selective profile:
SUPERIOR PHARMACOKINETICS
Improved drug-like properties and oral bioavailability to enable complete target coverage in tumors.
Non-covalent KRAS(ON+OFF) INHIBITION
Exquisite activity against slow-cycling mutations (G12V) requires inhibition of the KRAS(ON) state.
POTENCY AGAINST MULTIPLE KRAS MUTATIONS
High potency against >90% of all KRAS mutations found in cancer, including G12V and G12D.
SELECTIVITY OVER OTHER RAS ISOFORMS
Maximized tolerability and safety by sparing NRAS & HRAS to preserve normal cell function.
Clinical trial for KRAS mutations
Study sites are currently enrolling patients with advanced solid tumors with KRAS mutations. The purpose of this study is to characterize the safety and tolerability of ALTA3263 in adults with advanced solid tumor malignancies with KRAS mutations.
For patients and caregivers to learn more about the 3263-001 study, please visit our trial site.
Please contact clinical.trials@alterome.com for more information.